Ammonium carbamate in CO2 / NH3 Cascade refrigeration systems
The desire to use energy-efficient natural refrigerants while complying with safety requirements in food factories has led to increasing use of CO2/NH3 Casade systems. An ammonium carbamate sensor from HB Products can detect leaks of CO2 into ammonia, saving costly repairs and downtime.

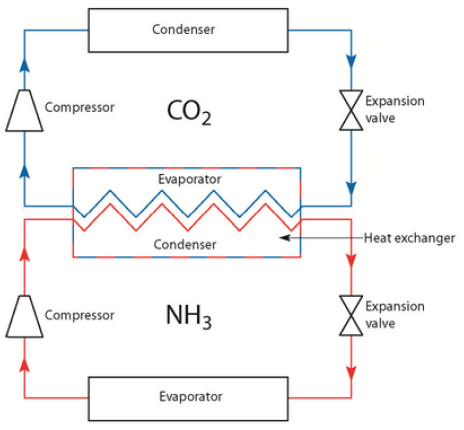
Cascade systems
A cascade system is a multi-stage refrigeration cycle where the evaporator of the first step is used as the condenser of the second step. When using climate-friendly natural refrigerants, the refrigerant in the first stage is often ammonia (NH3) and the second-stage refrigerant is CO2.
What happens in case of a leakage?
The operating pressure in the CO2 cycle is significantly higher than in the NH3 cycle. Therefore, a leakage in the heat exchanger can cause CO2 to enter the NH3 part of the system.
If not detected very quickly, a leak of CO2 into NH3 will cause a chemical reaction that creates ammonium carbamate, a corrosive salts. These salts can block valves, pipes and the rest of the system when transported with the flow of refrigerant. The salts may also accumulate in the evaporator and reduce the heat-transfer efficiency.
Operators are keen to shut down systems before these salts reach the compressors, as this can damage them and result in very costly downtime.
The leaks often happen in plate heat exchangers. These can rarely be repaired, but can be replaced, so the system can be put back into operation quickly .
The solution Creative Design
To solve this problem, HB Products has developed a sensor that detects ammonium carbamate. It is placed in the liquid ammonia, where it detects changes in permittivity
(dielectric constant), meaning how well the liquid conducts electric signals. The permittivity of ammonium carbamate is much higher than ammonia.
The sensor is very fast, detecting the rapid increase in conductivity/permittivity the moment CO2 enters the NH3 system.
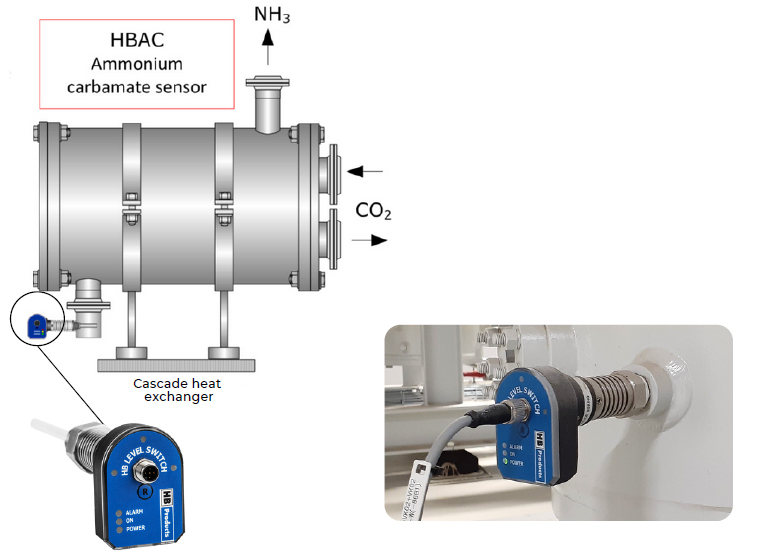
The sensor can deliver a digital alarm signal, or an emergency stop in the NH3 cycle, shutting down the system before the ammonium carbamate blocking the system.
Ammonium carbamate can be removed from the system mechanically, or by using warm air, but this is very time consuming, meaning costly downtime. Installing the HB Products sensor can therefore give very early warning of potential leaks before damage occurs to the rest of the system, saving money and time.
How is ammonium carbamate created?
The chemical reaction occurring when CO2 leaks into ammonia is:
CO2 + 2 NH3 → NH2COONH4
This means that one mole CO2 and two mole NH3 produce one mole ammonium carbamate (NH2COONH4). Converted into mass, this means that approximately 44g carbon dioxide and 34 g ammonia produce 78 g ammonium carbamate. At -10 °C and a pressure of 26.49 bar (boiling point) 44 g carbon dioxide in the gas phase takes up a volume of approximately 0.8 L. Crystalline ammonium carbamate has a density of 1.6 g/cm3 which – with the mentioned 78 g – takes up nearly 50 mL. Although this calculation is a good starting point, you shouldn’t assume that – in case of a leakage – only 0.8 L CO2 will enter the NH3 cycle.
Case Studies:
Ammonium carbamate in CO2/NHcascade refrigeration systems(1.25MB)





![White Paper : Vapor Quality Sensors [Before 2022]](http://www.hbtransducer.com/blog/zb_users/upload/2023/12/202312291703900198460949.jpg)