Detection of ammonia leakage into brine
How to use a pH sensor for detection of ammonia leakage into brine ?
When ammonia leak into brine the pH value will increase and this can be detected by the pH sensor. The sensor is suitable for surveillance of the pH value of most brines used in chillers and refrigeration systems.

Different chemical additions are used to lower the freezing point of the water, but they will also impact the pH value when ammonia leak into the brine. Salt like NaCl and potassium salts will act as a buffer and slow down the reaction, whereas glycol and ethanol has no impact on the pH development.
Sidenote: The senor cannot be used in systems without water like hydrocarbon liquids. For other liquids please contact the supplier of the brine or HB Products for more information.
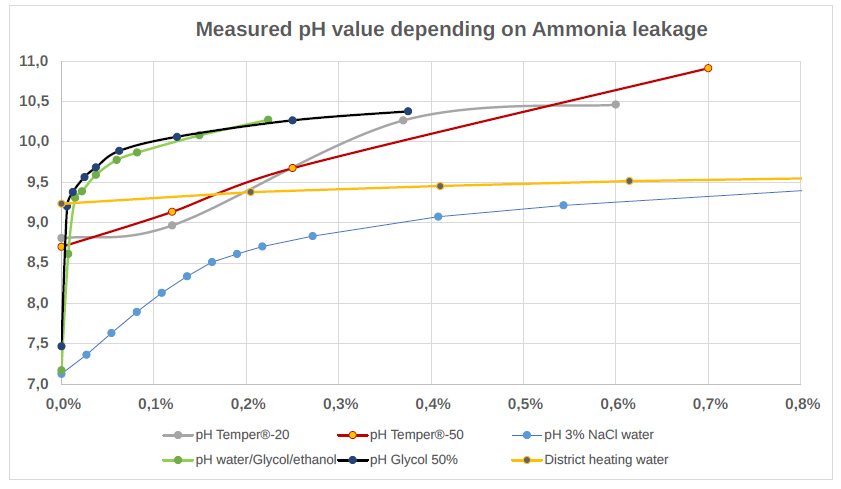
In fresh water and mixtures of water, ethanol, methanol, and glycol, a leakage of 1ml ammonia/It will increase the pH value rapidly from 7 to 10.
Brines using NaCl will still start at around pH7, and react slower than pure water, but there is still a clear reaction. For brine systems using potassium formate or potassium acetate, the pH value is above 8 to start with and the reaction is similar to brines with NaCl. The diagram shows measured values for how the pH value develops when ammonia is added to different brines.
Freshwater has the same curve as the glycol/ethanol/water mixtures.
Temper® is a brine from Temper Technologies which use potassium formate or potassium acetate to lower the freezing point and the have a pH value be- tween 8.5 and 9.
The HBPH is a two wire sensor with an 4-20 mA analog output. The sensor is suited for temperatures down to –15°C (5F). For temperatures below this the sensor need to installed in a bypass where you have to heat the brine passing the sensor.

Alarm and warnings
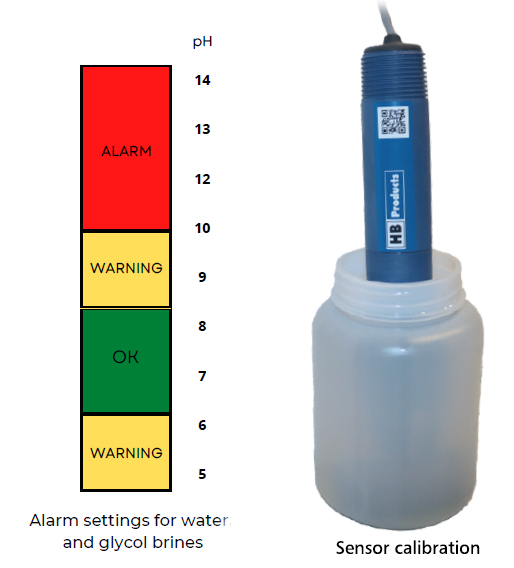
A pH sensor will change calibration over time depending on the fluid it is operating in. If you like to get a warning when the sensor have dried in calibration you can setup a two level warning/alarm system.
A two level warning/alarm system provide a warning when you need to check your sensor calibration and a second level where you stop due to leakage. If you have a base pH value of 7 it will make sense to use a warning for pH above 8 and pH below 6 . The second level can then be an alarm at pH=9. With such a system it is not necessary to make frequent checks and calibrations, however we recommend an annual check of the calibration.
For other brine systems like those using NaCl , potassium formate or potassium acetate it is more complicated and the limits have to be different.
The need for calibration
When delivered the sensor will have a basic calibration. This means that the sensor have to calibrated if you need a precise and accurate output. If you like to use the sensor for detecting ammonia leakage into fresh water of mix-tures between water and ethanol/methanol/glycol a calibration is normally not needed. If you like to use the sensor in other brines with a higher pH value or in NaCl brines, we strongly recommend a calibration. The reason is that an ammonia leakage will not impact the pH value as much in these fluids, because they act as a buffer.
How to check functionality
If you like to check the functionality in pure fresh water or fresh water mixed with ethanol/methanol/glycol it is simple - you can just dip the sensor into a 0.1% solution of ammonia into water as shown in the photo.






![White Paper : Vapor Quality Sensors [Before 2022]](http://www.hbtransducer.com/blog/zb_users/upload/2023/12/202312291703900198460949.jpg)